EFFECT OF DIFFERENT PRESERVATIVES ON THE HUMAN SOFT TISSUES STORED AT DIFFERENT TEMPERATURES AND INTERVALS OF TIME FOR THE ISOLATION OF DNA FOR DNA FINGERPRINTING
Anupuma Raina, Bhuvnesh K.Yadav, S Lalwani & TD Dogra
DNA Fingerprinting Laboratory, Deptt. of Forensic Medicine, All India Institute of Medical Sciences, New Delhi, India
Abstract:
The major problem faced by the forensic laboratories is to get the samples in proper conditions. Most of the biological samples brought for DNA analysis are either partially degraded or completely degraded and many a times it is not possible to extract DNA from them. It necessitates the optimization of the temperature conditions and preservatives for the storage of the biological samples (soft tissues). Our study concluded that -80°C temperature and normal saline as preservative is the best combination for the storage of the forensic samples till processed further. Since –80°C facility is not available in all the labs, the samples stored in normal saline at lower temperature (4°C) is found to be the feasible condition for long storage. Formalin fixation of the samples for long storage is not desirable for the isolation of DNA because first it has to be deformalized and also it hampers the PCR activity.
Key Words:- DNA fingerprinting, Preservatives, Time interval
INTRODUCTION
Rapid technological changes have allowed highly discriminating DNA profiling to be accomplished using trace samples found at crime scenes (1). These laboratory methods include techniques for DNA amplification, fragment separation and direct sequencing.
Besides fresh samples, evidences from the scene of crime are forwarded in form of dried or preserved in different preservatives and sometimes associated with contaminants (2). Raina & Dogra have discussed the procedure for collection and preservation of biological samples for DNA analysis (3). Postmortem degradation of DNA is a complex phenomenon, begning with autolysis and followed by aerobic and bacterial distruction of the cells (4). Physical and chemical degradation can distroy most of the DNA contained in the ancient remains (5). The high risk of contamination is due to the fact that DNA is highly degraded and only minute amounts are preserved. Left DNA is in poor condition, damaged and broken in small pieces causing difficulty in analysis (6). The high risk of contamination is due to the fact that ancient DNA is highly degraded and only minute amounts are preserved, while the PCR technique is extremely sensitive and can easily pick up tiny amounts of contaminant DNA (7). Contamination controls and detection therefore becomes extremely important in ancient DNA studies (8). It has been shown that the yield of DNA varies when seminal stain is stored on different textures of cloths (9).
DNA is susceptible to degradation by hydrolytic and oxidative endonuclease (10). By the time the samples reach to the DNA labs, samples are either degraded or decomposed completely. So one has to optimize the conditions at which the samples are received in the best form. But not any one condition is responsible for the degradation of DNA. It depends upon the condition of tissues, durations and temperature of storage and preservative used (11). Samples are mostly preserved by a large number of chemicals. Storage time, solution, type of tissues and storage temperature may affect the quantity and quality of the DNA. This work was carried out with the objectives to study best preservatives and effect of preservatives on DNA and to study effect of temperature, duration on the DNA.
MATERIAL AND METHODOLOGY:
Soft tissues (brain, kidney and heart) were collected from the unclaimed dead bodies from the mortuary, deptt. of Forensic Medicine and Toxicology AIIMS with prior permission of concern authority. The samples were cut into small pieces, weighed and stored in sterile containers having different preservatives (i.e. formalin, normal saline and tap water). These were stored at different temperature i.e. –80°C, -20°C, 4°C, room temperature and environmental conditions for different durations (i.e.1day, 2 days and so on upto11 weeks). The samples were observed for their physical conditions and then processed for the DNA isolation (12). The quantification of DNA was done using Agarose gel electrophoresis and spectroscopy.
RESULTS
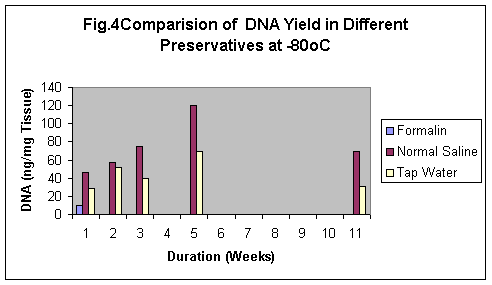
In this study, -80°C was observed the best storage condition temperature for the analysis of DNA. It was found that samples stored at -80°C yielded the best DNA both qualitatively and quantitatively (Fig.4). 120ng of DNA was isolated from the sample stored at –80°C for one day and 70ng of DNA after 11 weeks as compared to the samples which were stored at 4°C (64ng, 2ng) or environmental conditions (30ng).
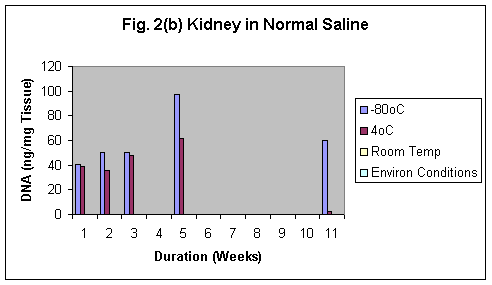
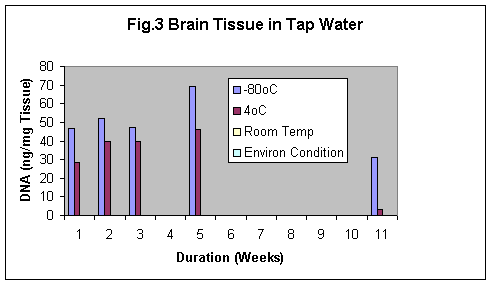
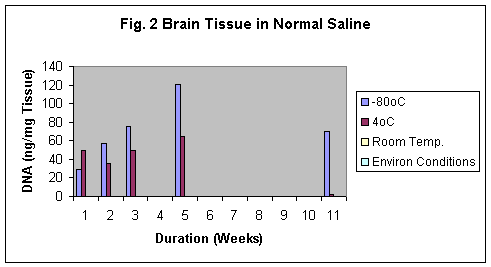
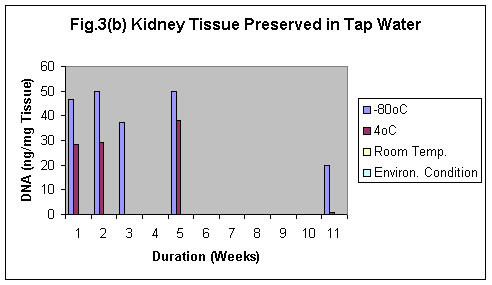
Normal saline was found to be the best preservative amongst formalin and tap water. The best results was observed using Normal saline even after 11 weeks in both brain and kidney samples (Fig.2 & 2b). Samples preserved in tap water showed the fastest degradation of the tissue (Fig 3 & 3b).
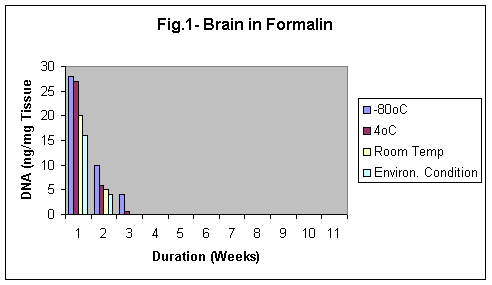
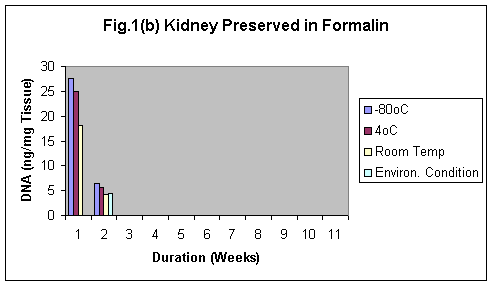
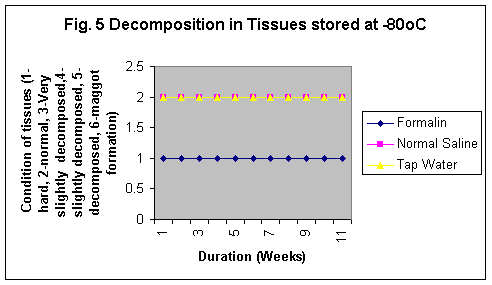
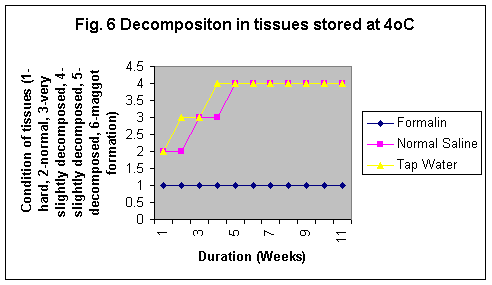
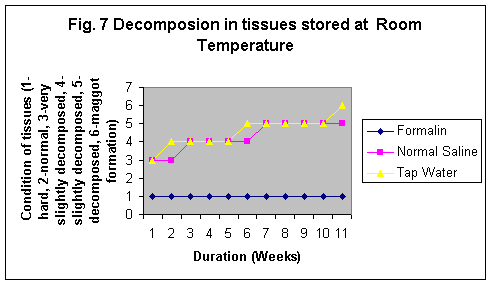
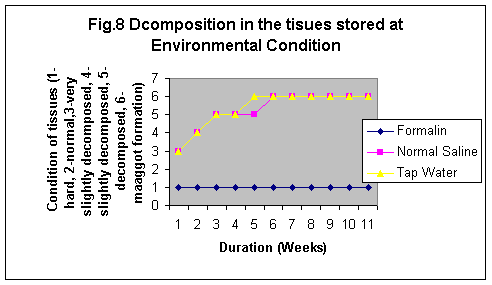
The samples stored in formalin although were least degraded (Fig. 5-8) but the yield of DNA was minimum (Fig 4, 1 & 1b). The reason may be that Formalin affects the quality of the DNA as it binds to the proteins.
In this study it was observed although the tissues stored in formaline become hard but do not degrade even stored for 11 wks at any temperature. However, Normal saline preservative shows degradation at RT and enviornmental condition even for short durations whereas at 40C the tissues were observed partially degraded after 5 weeks and the conditon of the sample was almost same even at 11th week. Tissues stored in tap water were observed as degraded after 2nd week but started developing maggot formation after 10 weeks at RT and in 5th week at enviornmental conditions. The condition of the tissues stored at -80°C in formailine, normal saline and tap water were almost after 11th week as was on the first day of storage (Fig. 5-8).
Isolation of DNA is inversely proportional to the duration of storage. But compared to the tissue type, storage solution and temperature, the duration of storage has minor effect on the DNA. On the basis of experimental observations and the gel results, it is concluded that – 80°C temperature and normal saline is the best combination for the long-term storage.
DISCUSSION
All the results of our 11 weeks study show that sample stored at -80°C in normal saline gave the best results. It is due to the fact that at –80°C degradation of sample is almost stopped and the samples remain as such. But as facility is not available in all the laboratories, therefore, it is not a feasible condition. The lower temperature i.e. at least is required with normal saline. 4°C also minimizes the rate of degradation but it is not for long-term storage. At the room temperature the samples are at their worst condition because at room temperature there is the fastest degradation the tissue. Even maggot formation occurs in the samples stored at environmental conditions. Earlier a study was conducted on the evaluation of DNA from blood samples stored at same temperature but for different duration of time. This study reveals that low temperature i.e. –70°C is the best storage condition but 4°C is most feasible condition for storage of samples (13).
Normal saline gives the isotonic condition to the samples, hence is the best preservative (Fig.4) whereas Formalin minimizes the tissue degradation to almost zero as it binds to the tissue proteins. Therefore, it is difficult to remove the protein and DNA isolated is usually contaminated with protein. Samples stored in formalin did not show maggot formation at any condition. Tap water is the worst preservative, as in almost all samples stored in tap water there was maggot formation.
Compared to the storage solutions and conditions of the storage, duration of the storage has minor affect on the degradation of the samples over the time course of the tissue study. As we can store samples at-80°C for indefinite time but in environmental conditions samples degrade in short durations i.e 1-2 weeks (Fig.4).
REFERENCE
| 1. | Raina A., Dogra T.D., (Aug 2002) Application of DNA Fingerprinting in Medico legal practice. JIMA, Vol.100 No.8, Page 1-8 |
| 2. | Lalji Singh (May 1991), DNA profiling and its applications, Current science Vol. 60, pp: 580-585 |
| 3. | Raina Anupuma & Dogra TD (2003) Methods of collection, preservation and forwarding of biological material for DNA fingerprinting. Indian Internet Journal of Forensic Medicne & Toxicology, vol. 1 No2. |
| 4. | Ludes B., Pfitzinger H. And Mangin P., (May 1993) DNA fingerprinting from tissues after variable postmortem periods. Journal of Forensic Science, JFSCA, Vol 38, No 3, pp: 686-690 |
| 5. | Yang DY., Watt K., (2005) Contamination control when preparing archaeological remains of ancient DNA analysis.” Journal of Archaeological Science, Vol 32, pp: 331-336 |
| 6. | Lindahl T., (1993) Instability and decay of the primary structure of DNA . Nature, Vol. 362 ,pp: 709-715 |
| 7. | O'Rourke DH, Hayes MG and Carlyle SW.,(2000) Ancient DNA studies in physical anthropology. Annual Review of Anthropology, Vol. 29, pp: 217-242. |
| 8. | Yang, D.Y. (2003) Contamination controls and detection in ancient DNA studies. Acta Anthropologica Sinica, Vol. 22, pp: 163-173. |
| 9. | Raina A, Pramanik P & Dogra TD (2005) Effect of storage conditions of seminal stains on different textures of cloths in relation to DNA yield. Indian Internet journal of Forensic Medicine & Toxicology, Vol.3 No.2 |
| 10. | Dessauer HC, Reeder TW, Cole CJ, Knight. (1996) A Rapid screening of DNA diversity using Dot-blot technology and Allele-specific oligonucleotides: Maternity of hybrids and unisexual clones of hybrid origin (Lizards, Cnemidophorus) Molecular Phylogenetics and Evolution, Vol. 6, pp: 366-372 |
| 11. | Shrinivasan M, Sedmak D, Jewell S., (2002) Effect of fixative and tissue processing on the contents and integrity of nucleic acids. American Journalof Pathology. Vol.161 No.6. |
| 12. | Sambrook and Fritsch (1989) Molecular Cloning: A laboratory manual.” Cold Spring Harbor Laboratory Press, IInd Edition |
| 13. | Ginya Bomjen, A Raina, IM Sulaiman, SE Hasnain & TD Dogra, (April 1996) Effect of storage of blood samples on DNA yeild, quality and fingerprinting: A Forensic Approach. Indian Journal of Experimental Biology, Vol.34, pp: 384-386 |
 |
 |
 |
 |
 |
 |
 |
 |
 |